What is corrosion?
Corrosion is an electrochemical process in which metals reacts with their surrounding environment. The resulting degradation leads to loss of material properties such as mechanical strength, appearance and impermeability to liquids and gases. The cause of corrosion is the difference in energy of metals and their natural ores. Energy is needed to extract any metal from its ore energy. It is this ‘excess energy’ which drives the corrosion as the metal will try to revert to its natural state.
Electrochemically, corrosion is a release of electrons. The reaction that gives away electrons is called oxidation and/or anodic reaction. However, these electrons need to be consumed somewhere and therefore a reduction or cathodic reaction needs to take place. In aqueous corrosion, the most common cathodic reactions are the reduction of dissolved oxygen or the evolution of hydrogen.
Metals that are easily oxidized, such as magnesium, are known as un-noble (reactive) metals while the most resistant ones, such as gold and platinum, are known as precious or noble metals. Most engineering metals, such as iron and copper, are found between these extremes.
Stainless steels and passivity
Stainless steels are not fundamentally noble materials in the same way as gold or platinum. Instead stainless steels derive their corrosion resistance from a thin, invisible and insoluble layer of chromium and iron oxides and hydroxides, commonly called the passive film, or passive layer (Figure 1).
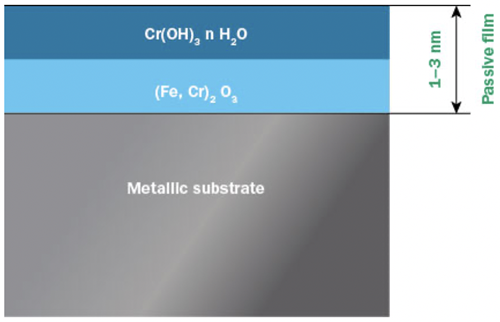
Figure 1: Two layer model of the passive layer on stainless steel.
Even though the passive layer is only a few nanometers in thickness, it effectively keeps the metal beneath isolated from its surroundings and electrochemical reactions causing corrosion are effectively slowed down. The passive layer reduces the corrosion rate to only a fraction of what it would be without it. Other metals such as chromium, aluminium and titanium also show passivity, and it is the ability of chromium to passivate that is utilized in stainless steels.
The passive layer on stainless steels forms spontaneously in environments containing enough oxidants. Moreover, if the metal beneath the passive layer is exposed by mechanical damage, such as scratches, it spontaneously repassivates. The oxygen content of air, and also of most aqueous solutions, is enough for both the creation and maintenance of the passive layer of stainless steels. If the passive layer is maintained, stainless steel can almost last forever.
Uniform corrosion
Uniform corrosion occurs in environments where the passive layer is not stable. The result is more or less uniform removal of metal from the unprotected surface, as shown in Figure 2. Uniform corrosion of stainless steels is most common in acids or hot alkaline solutions. However, molten chloride and fluoride salts may also cause uniform corrosion.

Figure 2: Uniform corrosion of a steam tube exposed to sulfuric acid.
In an environment with constant temperature and chemical composition, uniform corrosion is expected to occur at a reasonably constant rate. By knowing the surface area and measuring the weight loss over a period of time, the corrosion rate can be determined. This is often expressed as loss of thickness over time, e.g. mm/year. By definition stainless steel is normally considered to be resistant to uniform corrosion in a specific environment if the corrosion rate does not exceed 0.1 mm/year.
Pitting and crevice corrosion
While uniform corrosion causes widespread breakdown of the passive layer, pitting and crevice corrosion are caused by local breakdown of the passive layer. In practical situations, corrosion failure of stainless steel often occurs as the result of a localized attack rather than as the result of uniform corrosion, see Figure 3. In this case a galvanic couple is formed locally at the stainless steel surface which can lead to rapid propagation of corrosion. The weight loss can be very small compared to that experienced for uniform corrosion and expressing the severity of corrosion as corrosion rate is not relevant for localized attack. Instead pitting and crevice corrosion is approached as an either/or scenario where penetration of the material can take place very quickly once initiated and therefore needs to be avoided.

Figure 3: Pitting corrosion on the outside of a tube.
Stainless steels are particularly susceptible to pitting and crevice corrosion in environments with halide ions, e.g. chlorides. Therefore, environments posing a risk of localized corrosion include many types of water with high chloride content such as seawater and various process solutions.
Crevice corrosion, see Figure 4, occurs in crevices and other confined spaces, and also under deposits formed during service. The chemical reactions occurring naturally on a stainless steel surface in an aqueous environment consume oxygen. In the stagnant solution inside a crevice, the supply of new oxidant is restricted. The composition of the solution within the crevice can gradually become different from that of the ambient solution. This difference in composition increases the risk of corrosion, as a concentration cell is created.

Figure 4: Crevice corrosion under a flange sealing exposed to chlorinated seawater
The increasingly aggressive environment can eventually break down the passive layer inside the crevice and the small surface area of bare metal will behave as an anode to the much larger passive areas around the crevice.
As for any type of corrosion the risk of pitting and crevice corrosion depend on the combination of environmental factors and the corrosion resistance of the alloy. High chloride concentrations and low pH increase the probability of pitting and crevice corrosion, as do high temperatures. Other halides such as bromide and iodide may also cause pitting and crevice corrosion.
Stress corrosion cracking
Stress Corrosion Cracking (SCC) is a brittle failure mode caused by the combined effect of mechanical stress and a corrosive environment. The potential to cause rapid loss of mechanical strength with the potential result of fractures and catastrophic failures in environments where pitting, crevice or uniform corrosion is not expected makes it an insidious form of corrosion. For stress corrosion cracking to occur, three requirements must coincide:
- a susceptible material
- an environment in which the material is sensitive to SCC
- sufficient tensile stress
If one of these three factors is removed, SCC will not occur. As with pitting and crevice corrosion, stress corrosion of stainless steels is most frequently caused by solutions containing chloride and at elevated temperatures. The risk of SCC is increased with increasing chloride concentration, increasing temperature and decreasing pH. Stress corrosion attack on stainless steel typically takes the form of thin, branched cracks, see Figure 5.

Figure 5: Stress corrosion cracking of a stainless steel tube.
Failure caused by stress corrosion cracking often happens abruptly and without warning, due to the high propagation rates of the cracks. In the most severe cases, failure of a component may happen within a few days or even hours.
Corrosion fatigue
When a material is subjected to a cyclic load it can fail at loads far below the ultimate tensile stress of the material. If the material is simultaneously exposed to a corrosive environment, failure may take place at even lower loads and after shorter periods of time. This is caused by a form of corrosion known as corrosion fatigue, which has similarities to stress corrosion cracking causing brittle failures. However, cracks evolved from corrosion fatigue are less branched, see Figure 6.

Figure 6: Corrosion fatigue cracks in a paper machine suction roll shell.
Corrosion fatigue often takes place at ambient temperature and in moderately concentrated solutions that could be considered harmless with regard to other forms of corrosion.
Intergranular corrosion
lntergranular corrosion means preferential corrosion of the grain boundaries of a material. In stainless steel, intergranular corrosion may occur as a consequence of the precipitation of chromium carbide or intermetallic phases. This type of corrosion was previously a potential risk for stainless steel because of the high carbon content (0.05–0.15 %). Modern steel production methods, and especially the use of the AOD (argon oxygen decarburisation) converter, have enabled lower carbon contents with the result that intergranular corrosion is rarely a problem today.
Galvanic corrosion
When two dissimilar materials are connected electrically and immersed in a conductive liquid, an electrolyte, their corrosion performance might differ significantly when compared with the uncoupled metals. As a rule, the less noble material, the anode, is more severely attacked, whilst the more noble metal, the cathode, is essentially protected from corrosion. This galvanic corrosion attack is normally most evident close to the junction of the two metals, see Figure 7.

Figure 7: Galvanic corrosion of galvanized bolts connected to stainless steel.
As long as stainless steels stay passive, they are in most environments more noble than other metallic construction materials and therefore form the cathode in most galvanic couples. Galvanic coupling to stainless steels might, on the other hand, increase the corrosion rates of less noble metals such as mild steel, galvanized steel, copper and brass. Galvanic corrosion between different stainless steel is generally not a problem, provided that each stainless steel would be passive if exposed and uncoupled in the particular environment.
